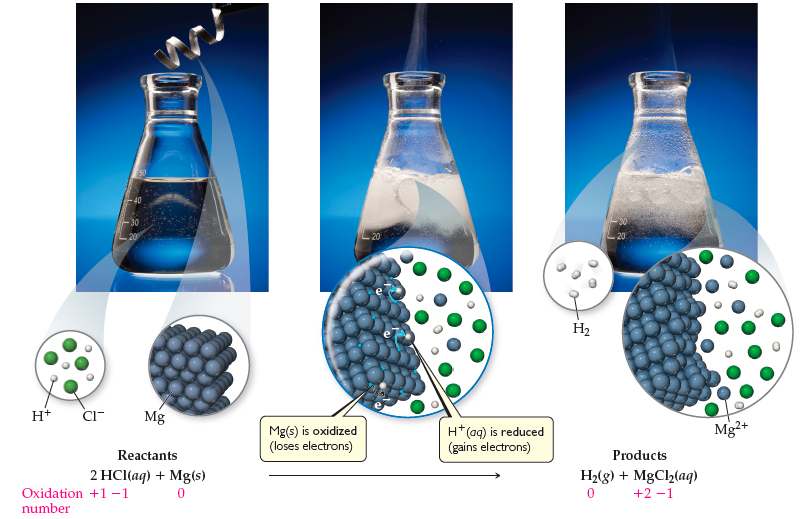
Hydrochloric Acid To Produce Hydrogen Gas . The electrolysis of water (h 2 o) is one of the easiest way to make hydrogen. Acids react with metals to produce a salt and hydrogen. Acid + metal → salt + hydrogen hydrochloric. This reaction will yield a. hydrogen chloride, a compound of the elements hydrogen and chlorine, a gas at room temperature and pressure. Acid + metal → salt + hydrogen. students follow the rate of reaction between magnesium and the acid, by measuring the amount of gas produced at 10 second intervals. in this experiment, students react magnesium ribbon with dilute hydrochloric acid to produce hydrogen gas. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. another method to chemically generate gas is the oxidation of metals in acidic solutions. They can then use the measured volume of.
from www.chegg.com
The electrolysis of water (h 2 o) is one of the easiest way to make hydrogen. Acid + metal → salt + hydrogen hydrochloric. Acids react with metals to produce a salt and hydrogen. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. This reaction will yield a. Acid + metal → salt + hydrogen. They can then use the measured volume of. in this experiment, students react magnesium ribbon with dilute hydrochloric acid to produce hydrogen gas. students follow the rate of reaction between magnesium and the acid, by measuring the amount of gas produced at 10 second intervals. another method to chemically generate gas is the oxidation of metals in acidic solutions.
Solved How many moles of hydrogen gas would be produced for
Hydrochloric Acid To Produce Hydrogen Gas acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. The electrolysis of water (h 2 o) is one of the easiest way to make hydrogen. students follow the rate of reaction between magnesium and the acid, by measuring the amount of gas produced at 10 second intervals. Acid + metal → salt + hydrogen. They can then use the measured volume of. Acids react with metals to produce a salt and hydrogen. This reaction will yield a. in this experiment, students react magnesium ribbon with dilute hydrochloric acid to produce hydrogen gas. Acid + metal → salt + hydrogen hydrochloric. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. hydrogen chloride, a compound of the elements hydrogen and chlorine, a gas at room temperature and pressure. another method to chemically generate gas is the oxidation of metals in acidic solutions.
From www.numerade.com
In this problem, zinc reacts with hydrochloric acid to make zinc Hydrochloric Acid To Produce Hydrogen Gas The electrolysis of water (h 2 o) is one of the easiest way to make hydrogen. Acid + metal → salt + hydrogen hydrochloric. This reaction will yield a. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. hydrogen chloride, a compound of the elements hydrogen and chlorine, a gas. Hydrochloric Acid To Produce Hydrogen Gas.
From classnotes.org.in
Chapter 2 Acids, Bases and Salts Class 10, NCERT Solutions, Science Hydrochloric Acid To Produce Hydrogen Gas Acid + metal → salt + hydrogen hydrochloric. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. students follow the rate of reaction between magnesium and the acid, by measuring the amount of gas produced at 10 second intervals. hydrogen chloride, a compound of the elements hydrogen and chlorine,. Hydrochloric Acid To Produce Hydrogen Gas.
From ceprltkf.blob.core.windows.net
Chlorine Gas Can Be Produced In The Laboratory By Adding Concentrated Hydrochloric Acid To Produce Hydrogen Gas Acid + metal → salt + hydrogen hydrochloric. Acids react with metals to produce a salt and hydrogen. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. The electrolysis of water (h 2 o) is one of the easiest way to make hydrogen. They can then use the measured volume of.. Hydrochloric Acid To Produce Hydrogen Gas.
From www.knowledgeboat.com
In the laboratory preparation of hydrochloric acid, hydrogen Hydrochloric Acid To Produce Hydrogen Gas students follow the rate of reaction between magnesium and the acid, by measuring the amount of gas produced at 10 second intervals. Acid + metal → salt + hydrogen hydrochloric. They can then use the measured volume of. in this experiment, students react magnesium ribbon with dilute hydrochloric acid to produce hydrogen gas. Acids react with metals to. Hydrochloric Acid To Produce Hydrogen Gas.
From www.chegg.com
Solved Aluminum reacts with concentrated hydrochloric acid Hydrochloric Acid To Produce Hydrogen Gas Acids react with metals to produce a salt and hydrogen. They can then use the measured volume of. Acid + metal → salt + hydrogen. students follow the rate of reaction between magnesium and the acid, by measuring the amount of gas produced at 10 second intervals. Acid + metal → salt + hydrogen hydrochloric. This reaction will yield. Hydrochloric Acid To Produce Hydrogen Gas.
From www.numerade.com
SOLVED Magnesium metal reacts with hydrochloric acid to produce Hydrochloric Acid To Produce Hydrogen Gas Acid + metal → salt + hydrogen. in this experiment, students react magnesium ribbon with dilute hydrochloric acid to produce hydrogen gas. They can then use the measured volume of. another method to chemically generate gas is the oxidation of metals in acidic solutions. Acids react with metals to produce a salt and hydrogen. This reaction will yield. Hydrochloric Acid To Produce Hydrogen Gas.
From fphoto.photoshelter.com
science chemistry exothermic reaction hydrochloric acid aluminum Hydrochloric Acid To Produce Hydrogen Gas Acid + metal → salt + hydrogen. The electrolysis of water (h 2 o) is one of the easiest way to make hydrogen. Acid + metal → salt + hydrogen hydrochloric. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. another method to chemically generate gas is the oxidation of. Hydrochloric Acid To Produce Hydrogen Gas.
From www.numerade.com
SOLVED Zinc dissolves in hydrochloric acid to yield hydrogen gas as Hydrochloric Acid To Produce Hydrogen Gas This reaction will yield a. Acid + metal → salt + hydrogen. another method to chemically generate gas is the oxidation of metals in acidic solutions. in this experiment, students react magnesium ribbon with dilute hydrochloric acid to produce hydrogen gas. acids will react with reactive metals, such as magnesium and zinc, to make a salt and. Hydrochloric Acid To Produce Hydrogen Gas.
From www.numerade.com
SOLVED Solid magnesium reacts with hydrochloric acid (HCI) to form Hydrochloric Acid To Produce Hydrogen Gas They can then use the measured volume of. in this experiment, students react magnesium ribbon with dilute hydrochloric acid to produce hydrogen gas. Acid + metal → salt + hydrogen. hydrogen chloride, a compound of the elements hydrogen and chlorine, a gas at room temperature and pressure. Acids react with metals to produce a salt and hydrogen. . Hydrochloric Acid To Produce Hydrogen Gas.
From www.numerade.com
SOLVED Water Vapor Temperature Pressure (mmHg) Magnesium metal is Hydrochloric Acid To Produce Hydrogen Gas acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. The electrolysis of water (h 2 o) is one of the easiest way to make hydrogen. Acid + metal → salt + hydrogen hydrochloric. students follow the rate of reaction between magnesium and the acid, by measuring the amount of gas. Hydrochloric Acid To Produce Hydrogen Gas.
From www.numerade.com
SOLVED Magnesium metal reacts with hydrochloric acid to produce Hydrochloric Acid To Produce Hydrogen Gas Acid + metal → salt + hydrogen hydrochloric. Acids react with metals to produce a salt and hydrogen. They can then use the measured volume of. students follow the rate of reaction between magnesium and the acid, by measuring the amount of gas produced at 10 second intervals. Acid + metal → salt + hydrogen. in this experiment,. Hydrochloric Acid To Produce Hydrogen Gas.
From www.numerade.com
SOLVED Based on the activity series, which metal dissolves in Hydrochloric Acid To Produce Hydrogen Gas acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. another method to chemically generate gas is the oxidation of metals in acidic solutions. Acids react with metals to produce a salt and hydrogen. students follow the rate of reaction between magnesium and the acid, by measuring the amount of. Hydrochloric Acid To Produce Hydrogen Gas.
From www.numerade.com
SOLVED aluminium reacts with hydrochloric acid to produce hydrogen gas Hydrochloric Acid To Produce Hydrogen Gas They can then use the measured volume of. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. in this experiment, students react magnesium ribbon with dilute hydrochloric acid to produce hydrogen gas. Acid + metal → salt + hydrogen hydrochloric. This reaction will yield a. Acids react with metals to. Hydrochloric Acid To Produce Hydrogen Gas.
From www.chegg.com
Solved How many moles of hydrogen gas would be produced for Hydrochloric Acid To Produce Hydrogen Gas students follow the rate of reaction between magnesium and the acid, by measuring the amount of gas produced at 10 second intervals. another method to chemically generate gas is the oxidation of metals in acidic solutions. hydrogen chloride, a compound of the elements hydrogen and chlorine, a gas at room temperature and pressure. This reaction will yield. Hydrochloric Acid To Produce Hydrogen Gas.
From www.alamy.com
Fully labelled diagram of the laboratory preparation of hydrogen Hydrochloric Acid To Produce Hydrogen Gas Acid + metal → salt + hydrogen. in this experiment, students react magnesium ribbon with dilute hydrochloric acid to produce hydrogen gas. students follow the rate of reaction between magnesium and the acid, by measuring the amount of gas produced at 10 second intervals. another method to chemically generate gas is the oxidation of metals in acidic. Hydrochloric Acid To Produce Hydrogen Gas.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Hydrochloric Acid To Produce Hydrogen Gas hydrogen chloride, a compound of the elements hydrogen and chlorine, a gas at room temperature and pressure. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. in this experiment, students react magnesium ribbon with dilute hydrochloric acid to produce hydrogen gas. Acids react with metals to produce a salt. Hydrochloric Acid To Produce Hydrogen Gas.
From www.numerade.com
SOLVED 1a) Write a balanced equation for the reaction of magnesium Hydrochloric Acid To Produce Hydrogen Gas hydrogen chloride, a compound of the elements hydrogen and chlorine, a gas at room temperature and pressure. Acid + metal → salt + hydrogen hydrochloric. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. in this experiment, students react magnesium ribbon with dilute hydrochloric acid to produce hydrogen gas.. Hydrochloric Acid To Produce Hydrogen Gas.
From www.numerade.com
SOLVED Zinc metal reacts with excess hydrochloric acid to produce Hydrochloric Acid To Produce Hydrogen Gas Acids react with metals to produce a salt and hydrogen. This reaction will yield a. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. They can then use the measured volume of. students follow the rate of reaction between magnesium and the acid, by measuring the amount of gas produced. Hydrochloric Acid To Produce Hydrogen Gas.